Instructions for Use: O2IN
Инструкция
This instruction manual applies to O2IN products in the United States. Version A – 2021-06, Approved Version Date: June 1, 2021.
O2IN® is a registered trademark of O2IN LLC in the United States.
Copyright © 2021 O2IN LLC
BIRDY BIRDS LLC 30 N GOULD ST STE R SHERIDAN WY 82801 UNITED STATES
Unauthorized reproduction of any part of this documentation, in any form, is prohibited without prior written consent from O2IN LLC. This includes processing, duplication, translation, or distribution using electronic systems. All rights reserved. Technical and graphical modifications, as well as typographical errors, are possible.
Производитель
BIRDY BIRDS LLC 30 N GOULD ST STE R SHERIDAN WY 82801 UNITED STATES
Contact Information
For any information about the product, in case of issues, or if you have any questions about its use, please contact the manufacturer's service center. Email: info@o2in.ru
Congratulations on purchasing the O2IN trainer!
The O2IN trainer creates oscillating positive pressure on exhalation and is designed to stimulate mucus clearance and strengthen respiratory muscles.
The O2IN can be used both at home and in professional sports and recreation facilities.
The device should only be used by individuals who understand the contents of the user manual and can safely and confidently operate the device. The methodology for use should be determined by qualified medical personnel based on individual needs.
This instruction manual applies to O2IN products in the United States. Version A – 2021-06, Approved Version Date: June 1, 2021.
O2IN® is a registered trademark of O2IN LLC in the United States.
Copyright © 2021 O2IN LLC
BIRDY BIRDS LLC 30 N GOULD ST STE R SHERIDAN WY 82801 UNITED STATES
Unauthorized reproduction of any part of this documentation, in any form, is prohibited without prior written consent from O2IN LLC. This includes processing, duplication, translation, or distribution using electronic systems. All rights reserved. Technical and graphical modifications, as well as typographical errors, are possible.
Производитель
BIRDY BIRDS LLC 30 N GOULD ST STE R SHERIDAN WY 82801 UNITED STATES
Contact Information
For any information about the product, in case of issues, or if you have any questions about its use, please contact the manufacturer's service center. Email: info@o2in.ru
Congratulations on purchasing the O2IN trainer!
The O2IN trainer creates oscillating positive pressure on exhalation and is designed to stimulate mucus clearance and strengthen respiratory muscles.
The O2IN can be used both at home and in professional sports and recreation facilities.
The device should only be used by individuals who understand the contents of the user manual and can safely and confidently operate the device. The methodology for use should be determined by qualified medical personnel based on individual needs.
Product Components
O2IN Basic
The product complies with Technical Specifications TU 323014 – 001 – 41396180 – 2019.
Composition:
Body: ABS plastic
Valve and mouthpiece: Silicone
Exhalation resistance valve: Stainless steel 440C
Additional resistances: Food-grade polystyrene
The materials comply with food industry standards.
Service Life and Manufacturer’s Warranty:
Service life of replaceable parts (mouthpiece, silicone valve, ball valve): 12 months
Service life of the device: 36 months
Manufacturer's warranty for plastic parts: 24 months
Manufacturer's warranty for silicone parts: 6 months
O2IN Basic
- Device body with mouthpiece and valve
- Key for opening side covers
- Storage pouch
- User manual
- Device body with mouthpiece and valve
- Moisture-retaining cover
- Set of resistances for inhalation and exhalation muscle training
- Key for opening side covers
- Storage pouch
- User manual
The product complies with Technical Specifications TU 323014 – 001 – 41396180 – 2019.
Composition:
Body: ABS plastic
Valve and mouthpiece: Silicone
Exhalation resistance valve: Stainless steel 440C
Additional resistances: Food-grade polystyrene
The materials comply with food industry standards.
Service Life and Manufacturer’s Warranty:
Service life of replaceable parts (mouthpiece, silicone valve, ball valve): 12 months
Service life of the device: 36 months
Manufacturer's warranty for plastic parts: 24 months
Manufacturer's warranty for silicone parts: 6 months
How O2IN Works
When you continuously exhale into the device, a vibration is generated towards the movable valve, which is transmitted to the lungs. This vibration helps to loosen mucus in the lower airways.
During use, the airways open up and the clearance of the loosened mucus is improved.
When you continuously exhale into the device, a vibration is generated towards the movable valve, which is transmitted to the lungs. This vibration helps to loosen mucus in the lower airways.
During use, the airways open up and the clearance of the loosened mucus is improved.


The device provides resistance during exhalation, which helps strengthen your respiratory muscles, increase lung capacity, and enhance the efficient use of oxygen during inhalation.
To adjust the device, open the right side cover using the provided key. Insert the key into the slot and turn it with force.
To adjust the device, open the right side cover using the provided key. Insert the key into the slot and turn it with force.
When the cover opens, you will see the adjustment handle. Exhale strongly and continuously through the device while turning the adjustment mechanism towards or away from yourself. Secure the handle in the position where you feel the maximum vibration during exhalation.


To train your inhalation muscles, open the cover and insert the resistance plate into the cover. The resistance plate can be installed on either side.
To replace the plate, push it out of the cover using the key.
To replace the plate, push it out of the cover using the key.
Using the Device
Remove the device from its packaging and carefully review the instructions.
Before use, thoroughly rinse the device under running water and wipe all parts with a soft, dry cloth.
Remember that the device is for individual use only; do not share it with others without changing the mouthpiece and disinfecting it.
Position the device as shown in the image. Do not turn the device upside down to ensure proper inhalation and exhalation:
• Place the mouthpiece between your teeth and seal it with your lips.
• Take a slow, deep breath in through the device or through your nose.
• Exhale deeply through your mouth into the device, trying not to puff out your cheeks if possible.
During exhalation, you should feel resistance.
Exhaling through the device should produce a distinctive sound and a sensation of vibration.
Remove the device from its packaging and carefully review the instructions.
Before use, thoroughly rinse the device under running water and wipe all parts with a soft, dry cloth.
Remember that the device is for individual use only; do not share it with others without changing the mouthpiece and disinfecting it.
Position the device as shown in the image. Do not turn the device upside down to ensure proper inhalation and exhalation:
• Place the mouthpiece between your teeth and seal it with your lips.
• Take a slow, deep breath in through the device or through your nose.
• Exhale deeply through your mouth into the device, trying not to puff out your cheeks if possible.
During exhalation, you should feel resistance.
Exhaling through the device should produce a distinctive sound and a sensation of vibration.

Possible Exercises Using the O2IN Device:
- Running (including on a treadmill);
- Walking, Nordic walking;
- Cycling;
- Rowing;
- Fitness, exercises on gym equipment;
- Walking or home activities (such as exercise, cleaning)
In the beginning, it's important to let your body adjust to the "interference" that affects normal breathing. Given the device’s design, it will encourage you to take deep breaths and exhale strongly and thoroughly, regardless of the exercise intensity.
Therefore, we recommend starting with static breathing exercises. After 3-4 minutes, you can move on to walking or arm movements.
When using the device statically (without movement), dizziness may occur due to hyperventilation. If this happens, you should stop the session or exercise immediately.
- Running (including on a treadmill);
- Walking, Nordic walking;
- Cycling;
- Rowing;
- Fitness, exercises on gym equipment;
- Walking or home activities (such as exercise, cleaning)
In the beginning, it's important to let your body adjust to the "interference" that affects normal breathing. Given the device’s design, it will encourage you to take deep breaths and exhale strongly and thoroughly, regardless of the exercise intensity.
Therefore, we recommend starting with static breathing exercises. After 3-4 minutes, you can move on to walking or arm movements.
When using the device statically (without movement), dizziness may occur due to hyperventilation. If this happens, you should stop the session or exercise immediately.
Do not rush into performing exercises with maximum intensity during your initial workouts. It is essential to get accustomed to breathing through the device.
For beginners, the recommended duration of using the device in a single session is 15-20 minutes with a heart rate not exceeding 110-120 beats per minute. After 4-5 sessions, depending on your comfort level, you may increase the usage time to 20-25 minutes per session, but not more than 30% of the total workout time.
For professionals, the usage time should be at least 35-40% of the total workout duration, with a heart rate of 120-140 beats per minute.
If you train 8-10 times a week, use the O2IN device between workouts. If you train 2-3 times a week, use the device during each session.
IMPORTANT! Individuals not prepared for high physical loads should avoid using the O2IN device when the heart rate exceeds 120 beats per minute.
For beginners, the recommended duration of using the device in a single session is 15-20 minutes with a heart rate not exceeding 110-120 beats per minute. After 4-5 sessions, depending on your comfort level, you may increase the usage time to 20-25 minutes per session, but not more than 30% of the total workout time.
For professionals, the usage time should be at least 35-40% of the total workout duration, with a heart rate of 120-140 beats per minute.
If you train 8-10 times a week, use the O2IN device between workouts. If you train 2-3 times a week, use the device during each session.
IMPORTANT! Individuals not prepared for high physical loads should avoid using the O2IN device when the heart rate exceeds 120 beats per minute.

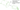
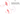
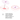




How to Open the Caps
How to Adjust the Angle of Operation
How to Set the Resistance
Assembly Instructions for the Trainer


WARNING!
The device has open parts and contains small components that can obstruct the airways and pose a choking hazard. All parts of the device should be kept out of reach of infants and young children.
Before each use, make sure that the device is free from any foreign objects or particles.
The device has open parts and contains small components that can obstruct the airways and pose a choking hazard. All parts of the device should be kept out of reach of infants and young children.
Before each use, make sure that the device is free from any foreign objects or particles.
PROHIBITED!
Do not use O2IN trainers outdoors when the air temperature is below 0°C. This can lead to throat and respiratory illnesses.
Do not use O2IN trainers outdoors when the air temperature is below 0°C. This can lead to throat and respiratory illnesses.
Cleaning and Maintenance
During training, saliva, mucus, and phlegm are produced, so it is essential to:
• After each use, rinse all parts under running water for 2 minutes. You may use a detergent (for dishwashing). Add 1 teaspoon of detergent to 0.5 liters of drinking water. Soak all individual parts in the cleaning solution for 5 minutes, then rinse under running water. Place all parts on a dry, clean, and absorbent surface and allow them to air dry completely;
• Use only clean and dry components. Contaminants and residual moisture can promote the growth of microorganisms, increasing the risk of infection;
• Ensure all individual parts are thoroughly dried after each cleaning step;
Do not store the components in a damp place or with wet items.
Store the trainer in the special pouch found in the packaging with the trainer and instruction manual.
Side Effects
Dizziness may occur. If dizziness appears, stop the training and do not continue until the dizziness completely resolves. Inform your doctor about this to discuss further use of the trainer.
Inform your doctor if any side effects occur that are not mentioned in this user manual.
During training, saliva, mucus, and phlegm are produced, so it is essential to:
• After each use, rinse all parts under running water for 2 minutes. You may use a detergent (for dishwashing). Add 1 teaspoon of detergent to 0.5 liters of drinking water. Soak all individual parts in the cleaning solution for 5 minutes, then rinse under running water. Place all parts on a dry, clean, and absorbent surface and allow them to air dry completely;
• Use only clean and dry components. Contaminants and residual moisture can promote the growth of microorganisms, increasing the risk of infection;
• Ensure all individual parts are thoroughly dried after each cleaning step;
Do not store the components in a damp place or with wet items.
Store the trainer in the special pouch found in the packaging with the trainer and instruction manual.
Side Effects
Dizziness may occur. If dizziness appears, stop the training and do not continue until the dizziness completely resolves. Inform your doctor about this to discuss further use of the trainer.
Inform your doctor if any side effects occur that are not mentioned in this user manual.
Contraindications
• Individual intolerance (e.g., severe gag reflex, allergies);
• Chronic and infectious diseases in the acute or decompensated stage;
• Respiratory insufficiency with significant hypoxemia combined with hypercapnia;
• Pneumothorax, recurrent pulmonary hemorrhage, and hemoptysis;
• Severe hypertensive crisis;
• Cancer patients during chemotherapy and for three months following treatment;
• Acute post-stroke or post-myocardial infarction phase (first 3 months after a stroke or heart attack);
• Anemia;
• Pregnancy after the 5th month;
• Post-operative recovery period (3 months after abdominal surgeries);
• Stage II or higher circulatory insufficiency;
• Angina pectoris, functional class III-IV;
• Severe cardiac rhythm and conduction disorders (e.g., paroxysms of tachysystolic atrial fibrillation or flutter occurring more than twice a month, supraventricular tachycardia with attacks more than twice a month, polymorphic and group extrasystole, atrioventricular block II-III degree, complete atrioventricular block);
• Grade III arterial hypertension;
• Cardiac aneurysm (acute or chronic) with symptoms of circulatory insufficiency greater than stage II A;
• Acute stage of cerebrovascular accident; parasitic diseases;
• Diseases with persistent pain syndrome requiring constant use of narcotic and psychotropic substances listed in Schedule I and II of the List of Narcotic Drugs, Psychotropic Substances, and Their Precursors Controlled in the Russian Federation, registered as medicinal products;
• Active tuberculosis of any localization (for non-tuberculosis sanatorium and spa institutions);
• Malignant tumors requiring antitumor treatment, including chemotherapy;
• Epilepsy with ongoing seizures, including those resistant to treatment;
• Epilepsy in remission for less than 6 months (for non-psychoneurological sanatorium and spa institutions);
• Mental and behavioral disorders in acute stages or unstable remission, including those posing a risk to the patient and others;
• Cachexia of any origin;
• For chronic diseases, the trainer should be used only during non-exacerbation periods.
• Individual intolerance (e.g., severe gag reflex, allergies);
• Chronic and infectious diseases in the acute or decompensated stage;
• Respiratory insufficiency with significant hypoxemia combined with hypercapnia;
• Pneumothorax, recurrent pulmonary hemorrhage, and hemoptysis;
• Severe hypertensive crisis;
• Cancer patients during chemotherapy and for three months following treatment;
• Acute post-stroke or post-myocardial infarction phase (first 3 months after a stroke or heart attack);
• Anemia;
• Pregnancy after the 5th month;
• Post-operative recovery period (3 months after abdominal surgeries);
• Stage II or higher circulatory insufficiency;
• Angina pectoris, functional class III-IV;
• Severe cardiac rhythm and conduction disorders (e.g., paroxysms of tachysystolic atrial fibrillation or flutter occurring more than twice a month, supraventricular tachycardia with attacks more than twice a month, polymorphic and group extrasystole, atrioventricular block II-III degree, complete atrioventricular block);
• Grade III arterial hypertension;
• Cardiac aneurysm (acute or chronic) with symptoms of circulatory insufficiency greater than stage II A;
• Acute stage of cerebrovascular accident; parasitic diseases;
• Diseases with persistent pain syndrome requiring constant use of narcotic and psychotropic substances listed in Schedule I and II of the List of Narcotic Drugs, Psychotropic Substances, and Their Precursors Controlled in the Russian Federation, registered as medicinal products;
• Active tuberculosis of any localization (for non-tuberculosis sanatorium and spa institutions);
• Malignant tumors requiring antitumor treatment, including chemotherapy;
• Epilepsy with ongoing seizures, including those resistant to treatment;
• Epilepsy in remission for less than 6 months (for non-psychoneurological sanatorium and spa institutions);
• Mental and behavioral disorders in acute stages or unstable remission, including those posing a risk to the patient and others;
• Cachexia of any origin;
• For chronic diseases, the trainer should be used only during non-exacerbation periods.